You may already know that handwashing with traditional soap is one of the best ways to get rid of bacteria and viruses. In case you are wondering why soap works so well, here is a quick explanation!
Soap is made of tiny molecules that are shaped much like a lollipop on a squiggly stick. The stick part of the molecule is called the “hydrophobic tail” since it does not like to be around water. It would prefer to be surrounded by oils and lipids (lipid is another word for fat). The round part of the lollipop, also known as the “hydrophilic head,” loves to surround itself and bond with water.
When soap comes into contact with water, sometimes a single lollipop shaped soap molecule will interact with other molecules; and at other times the soap molecules will congregate around a particle in a ring shaped bubble called a micelle. When a micelle forms, the water loving heads will always align toward the water on the outside with the tails facing inward toward the particle.
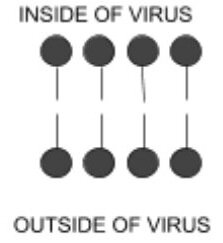
Many types of bacteria and viruses have a lipid outer layer that looks like a micelle but with a double wall. This is sometimes referred to as a phospholipid bilayer. Instead of a single ring of molecules, there are are two that are set in a mirror image, with the sticks facing each other. Organisms that have this two-layer outer membrane include coronaviruses, viruses that cause Hepatitis B, Hepatitis C, HIV, Ebola, Zika, Dengue Fever and also many types of bacteria.
In the case of some bacteria and viruses, there are also proteins and other factors sandwiched within the lipid membrane that allows the particle to adhere to a healthy cell and replicate itself.
When a single soap molecule comes near a particle such as the coronavirus, the water fearing tail of the soap molecule wedges itself into the lipid membrane of the virus in order to evade water. This wedge action prys apart the membrane, essentially spilling out all the necessary components that the virus needs to survive. Bye bye virus!
So, what happens to the remnants of the bacteria and viruses? Several of the soap molecules will surround the leftover fragments to form a micelle. (Those tails are still avoiding water!) The action of rubbing your hands to make soapy bubbles and then rinsing will carry these little micelles away. It’s that simple!
As a side note, hand sanitizers containing at least 60% alcohol will act in a similar manner by destabilizing the membranes of bacteria and viruses, but they are far less effective at removing the debris from your hands and body since they don’t make bubbly micelles. So wash with soap! It smells way better and will get you cleaner.